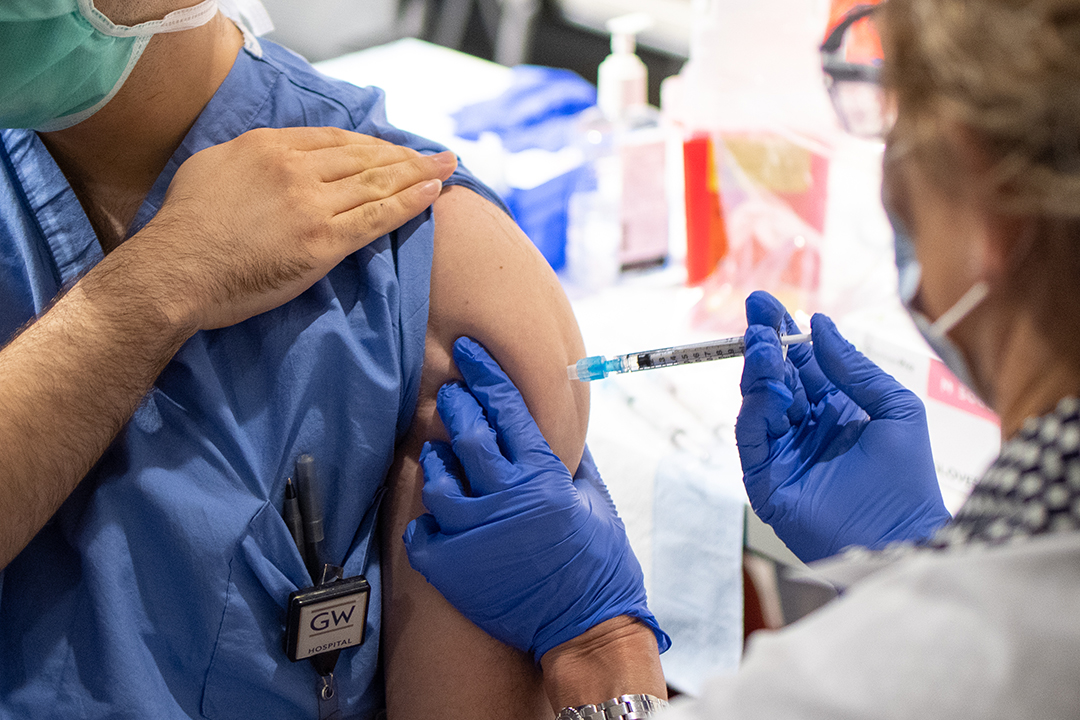
The George Washington University announced Wednesday its participation in a Sanofi COVID-19 vaccine clinical trial. GW was selected as one of approximately 25 sites in the United States to launch a phase 2 study for its adjuvanted recombinant protein-based COVID-19 vaccine candidate.
“Our research team at GW is proud to play a role in testing COVID-19 vaccine candidates to help end one of the greatest public health challenges of our time,” said David Diemert, principal investigator for the Sanofi COVID-19 vaccine trial at GW. “We know that in order to end this pandemic, we’ll need more than one approach to make sure we have enough effective vaccines available for everyone. We are excited to begin recruiting volunteers for the Sanofi COVID-19 vaccine trial in the coming weeks.”
Sanofi’s adjuvanted recombinant protein-based COVID-19 vaccine candidate, which is developed in partnership with GlaxoSmithKline, uses the same proven technology as Sanofi’s recombinant influenza vaccine. This vaccine candidate is similar in design to the newest shingles and hepatitis B vaccines, Dr. Diemert said. The backbone of this vaccine is a manufactured form of the SARS-CoV-2 spike protein that aims to stimulate the immune system to produce neutralizing antibodies against the virus. After being retooled in December 2020, Sanofi announced that new phase 2 testing would begin with a focus on safety and immune response.
GW plans to recruit 40 volunteers for the Sanofi COVID-19 vaccine trial. Volunteers must be at least 18 years old, with at least half to be over the age of 60. This will ensure the vaccine works across all ages, especially older individuals who have been disproportionately affected by COVID-19, Dr. Diemert said.
All volunteers will receive two doses of the vaccine, though certain groups will receive higher and lower amounts of the vaccine. The main results of the clinical trial are expected by the second quarter of 2021, although the study will continue through 2022. If results are positive, a phase 3 study looking at vaccine efficacy could begin in the second quarter of 2021. Positive results from the phase 3 study would lead to regulatory submissions in the second half of 2021, with the vaccine expected to be available in the fourth quarter of 2021, if approved.
This will be the second COVID-19 vaccine trial at GW. Dr. Diemert is also the principal investigator for a phase 3 study for Moderna’s COVID-19 vaccine. Dr. Diemert, who is a professor of medicine at the GW School of Medicine and Health Sciences and a physician in the Division of Infectious Diseases at the GW Medical Faculty Associates (GW MFA), will work alongside an interdisciplinary team of researchers at SMHS, GW MFA, and the Milken Institute School of Public Health at GW.
“We are thrilled with the success of the Moderna COVID-19 vaccine trial that we have been a part of, but recognize the critical need for additional vaccines. Participating in this trial will not only help advance the development of a different vaccine platform against COVID-19, but also give those volunteers who were not selected to participate in the Moderna trial the opportunity to contribute in the fight to end this pandemic,” said Marc Siegel, a co-investigator for the Sanofi COVID-19 vaccine trial at GW, associate professor of medicine at GW SMHS, and a physician in the Division of Infectious Diseases at the GW MFA.