You are anxious or sense danger approaching. Your sympathetic nervous system kicks into gear and releases a neurotransmitter, called norepinephrine, from your neurons, triggering a “fight or flight” response in the body.
Your palms sweat. Your breath quickens. Your blood sugar skyrockets.
To increase blood flow to your muscles, sympathetic fibers also release norepinephrine in your heart. It throbs. Faster and faster.
Usually, your heart rate will slow back to normal, but sometimes, all of that sympathetic activity can cause the heart to pump dangerously out of synch—or stop beating altogether.
“If you have an existing heart problem, a sudden increase in heart rate and force, motivated by the fight or flight response, can reveal it,” says Matt Kay, an associate professor of biomedical engineering in the School of Engineering and Applied Science.
While there seems to be a clear link between irregular heartbeats, known as arrhythmias, and the body’s response to severe stress, scientists still don’t fully understand the complex relationship.
Now, an interdisciplinary team of George Washington University engineers and physiologists are taking a deeper look at this phenomenon using optogenetics—a breakthrough technology that employs light to selectively manipulate neurons and physiological behavior in genetically altered animals.
“Our goal is to better understand the interaction between sympathetic neurons and heart tissue, and how it might lead to dangerous arrhythmias or sudden cardiac death,” says Anastasia Wengrowski, a Ph.D. student in the Department of Biomedical Engineering who led the project. “If you can better understand how, where and why the problem happens, then you can go about fixing it.”
Blending Biology and Technology
February is American Heart Month. Heart disease is the number one cause of natural death in the United States. Sudden cardiac arrest kills about 320,000 people per year, according to the Centers for Disease Control and Prevention.
While the two can be linked, sudden cardiac death differs from heart attacks, which often occur when arteries in the heart are blocked. By contrast, people experience cardiac arrest when the heart’s electrical system glitches and beats irregularly fast. Without immediate emergency treatment, death quickly follows.
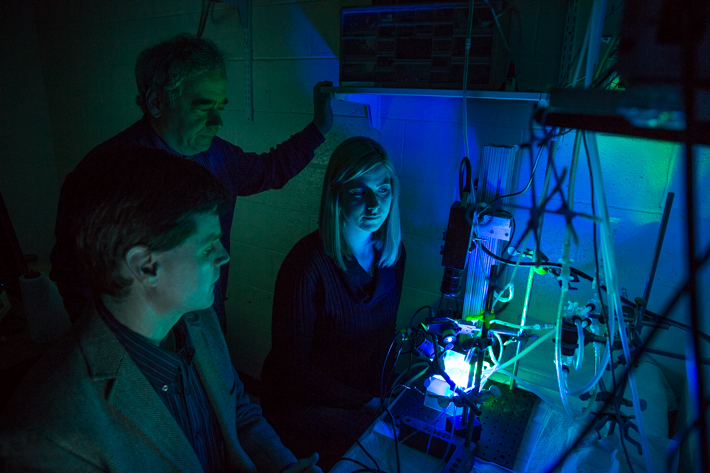
Dr. Kay has spent years studying the “tornadoes” of electricity that twist in the heart’s ventricles, a leading cause of sudden cardiac arrest. In his Ross Hall lab, researchers have assembled a system of tubing, pumps and cameras to keep alive the hearts of mice and rabbits.
Then, using LED lights, high-speed cameras and a fluorescent dye that illuminates the heart’s electrical activity, Dr. Kay and his team are able to image and record data, such as changes in heart rate and waves of depolarization flowing through the heart. The approach is called “optical mapping.”
David Mendelowitz, a professor of pharmacology and physiology in the School of Medicine and Health Sciences, studies how neurons in the brain control heart rate in health and disease. In his lab, researchers breed mice to express light-activated proteins in sympathetic neurons. They use these mouse models to study the role of brain regions in cardiovascular diseases.
During a racquetball game with Dr. Mendelowitz, Dr. Kay questioned whether he could use the genetically altered mice from his colleague’s lab to study heart arrhythmias, rather than brain activity.
“It was an overlap between our interests,” Dr. Kay says. “We thought it would be interesting to study whether this model created a condition where we could use light to activate sympathetic response in the heart.”
Pumping the Heart—With Light
When Ph.D. student Ms. Wengrowski shines blue LED lights on the transgenic mouse hearts, the hearts’ light-activated protein excites the sympathetic neurons, mimicking the heart’s fight or flight response to stress. Essentially, the researchers are able to control the heart’s response to sympathetic activity with a single switch.
While optogenetics is a hot topic in the neuroscience community, the GW researchers say it has rarely been used to study heart dysfunction.
“It’s a cutting edge idea. Optogenetics has been developed in the past five years in the neuroscience field. But it has not been applied extensively in cardiology,” Dr. Kay says.
Gathering data from the imaging system to detect changes in cardiac function, Ms. Wengrowski found that light caused the hearts to beat faster, contract more forcefully and made them more susceptible to arrhythmias. The results were published this winter in the journal Cardiovascular Research.
Past studies have used beta blockers, such as Propranolol, to study the same effect, but this approach does not allow researchers to study the heart’s sympathetic activity in a detailed way, Dr. Mendelowitz says. The way people usually study the sympathetic response to the heart is by adding catecholoamines, hormones made by the adrenal glands, to the blood or fluid.
“This bath solution goes everywhere in the heart. In that scenario, the agent is not released by the nerves as it normally occurs in the body,” he says. “What is unique about our model is that the sympathetic neurons can be selectively activated with light, without activating anything else at the same time, which more closely mimics what happens physiologically.”
Ms. Wengrowski says this new approach for studying sympathetic activity in the heart could eventually lead to better-targeted drugs and medical treatment. Next, the researchers plan to potentially inhibit, rather than stimulate sympathetic fibers, to see how that may reduce the risk of arrhythmias.
It’s not unlikely, Dr. Mendelowitz says, that doctors may one day cure arrhythmias with a luminous glow.
“This design is a first step for future studies,” Ms. Wengrowski says. “It characterizes how this phenomenon is working and defines the context for later repairing it.”